Temsirolimus Fda Approval History : Sequencing Therapies For Metastatic Renal Cell Carcinoma Urologic Clinics
FDA Approval for Temsirolimus Torisel for Advanced RCC. 1-888-info-fda 1-888-463-6332 Contact FDA Subscribe to FDA RSS feeds Follow FDA on Twitter Follow FDA on Facebook View FDA videos on YouTube View FDA photos on Flickr.
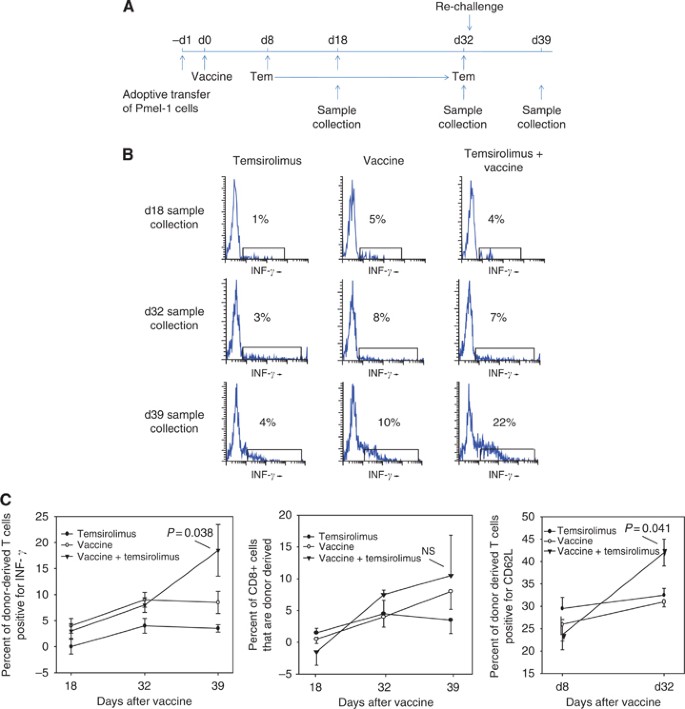
Temsirolimus An Mtor Inhibitor Enhances Anti Tumour Effects Of Heat Shock Protein Cancer Vaccines British Journal Of Cancer
Renal Cell Carcinoma Torisel temsirolimus an inhibitor of mTOR kinase is an antineoplastic agent indicated for the treatment of patients with advanced renal cell.
Temsirolimus fda approval history. Temsirolimus is approved to treat. TORISEL Kit temsirolimus injection for intravenous infusion only Initial US. Temsirolimus Torisel injection is a mammalian target of rapamycin mTOR inhibitor.
See full prescribing information for TORISEL. FDA Approves Temsirolimus for the Treatment of Advanced Kidney Cancer. FDA label information for this drug is available at DailyMed.
Advanced means that the cancer has started to spread. Food and Drug Administration FDAs approval of temsirolimus Torisel on May 30. Torisel is a medicine used to treat patients with the following types of cancer.
2007 -----INDICATIONS AND USAGE ----- TORISEL is a kinase inhibitor indicated for the. Yes First approved March 30 2009 Brand name. Initial FDA approval for the treatment of advanced renal cell carcinoma.
This report summarizes the US. The FDA has approved the use of temsirolimus for the treatment of advanced renal cell cancer RCC. Renal Cell Carcinoma Breast Cancer Neuroendocrine Carcinoma Afinitor everolimus is an oral once-daily inhibitor of mTOR indicated for the treatment of patients with advanced HR HER2- breast cancer.
Oncology ONCOLOGY Vol 21 No 7 Volume 21 Issue 7. TORISEL safely and effectively. It is approved in the United States for the treatment of advanced renal cell carcinoma.
Mantle cell lymphoma a cancer of B cells a type of white blood cell. Yes First approved May 30 2007 Brand name. Temsirolimus Torisel patient drug information Chemocare Temsirolimus Torisel patient drug information UpToDate History of changes in FDA indication.
Temsirolimus is an intravenous drug approved by the FDA for treatment of other cancers kidney cancer certain types of lymphoma but not for brain tumors. June 25th 2007 - Volume 29 - Issue 12 - p 58. Advanced renal cell carcinoma a kidney cancer.
The approval of temsirolimus for the treatment of metastatic RCC arises from the GLOBAL study an international multicenter open-label three-arm. After completing this course the reader will be able to. Information provided includes regulatory history study design study results and literature review.
A brief regulatory history regarding the pediatric development plan is presented in Table 1 below. It received FDA approval in 2007 for the treatment of advanced RCC as the first mTORC1 inhibitor 18. This article is available for continuing medical education credit at This report summarizes the US.
Temsirolimus as Treatment for Advanced Renal Cell Carcinoma VIRGINIA E. Temsirolimus is an intravenous drug for the treatment of renal cell carcinoma RCC developed by Wyeth Pharmaceuticals and approved by the FDA in late May 2007 and was also approved by the European Medicines Agency EMEA on November 2007. Afinitor FDA Approval History.
Currently temsirolimus is used for the treatment of advanced RCC with poor prognostic factor. Temsirolimus is also being studied in the treatment of other types of cancer. Torisel is used in adults when the lymphoma has come back after previous treatment.
Renal cell carcinoma a type of kidney cancer that is advanced. Torisel is approved for the treatment of adults with advanced renal cell carcinoma. Food and Drug Administration FDAs approval of temsirolimus Torisel on May 30 2007 for the treatment of advanced renal cell carcinoma RCC.
Novartis AG Treatment for. Torisel FDA Approval History. Perifosine is a pill that has not been approved by the FDA which blocks a messenger that tells cancer cells to grow.
The drug made by Wyeth Pharmaceuticals as Torisel is the first targeted. Temsirolimus Torisel package insert. Wyeth Pharmaceuticals recently announced that the US Food and Drug Administration FDA has approved temsirolimus Torisel for patients with advanced renal cell carcinoma RCC.
FARRELLa ROBERT JUSTICEa SHAN SUN MITCHELLb RAJESHWARI SRIDHARAa RICHARD PAZDURa aDivision of Drug Oncology Products Office of Oncology Drug Products Center for Drug Evaluation and Research US. PROWELLa AMNA IBRAHIMa ANN T.

Anticonvulsant Agents Everolimus Springerlink
Theoncologist Onlinelibrary Wiley Com

Rapid Developments In Past 10 Years In Treatment Of Renal Cell Carcinoma Download Scientific Diagram
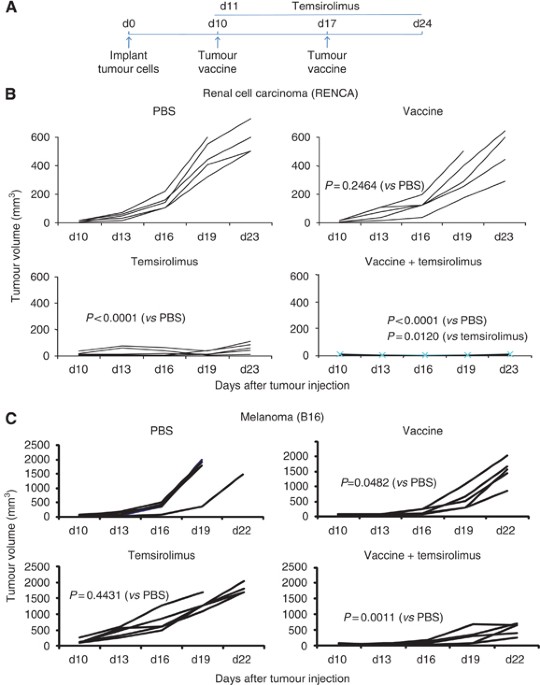
Temsirolimus An Mtor Inhibitor Enhances Anti Tumour Effects Of Heat Shock Protein Cancer Vaccines British Journal Of Cancer
Theoncologist Onlinelibrary Wiley Com

Fda Approval Summary Temsirolimus As Treatment For Advanced Renal Cell Carcinoma Abstract Europe Pmc

Sequencing Therapies For Metastatic Renal Cell Carcinoma Urologic Clinics

Mechanistic Target Of Rapamycin Inhibitors Successes And Challenges As Cancer Therapeutics
Theoncologist Onlinelibrary Wiley Com
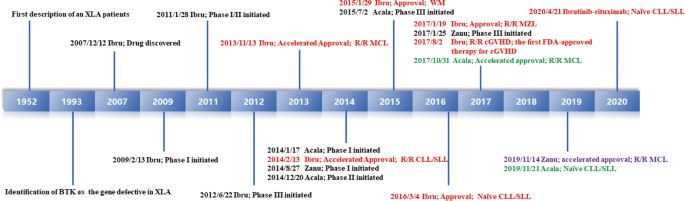
Inhibitors Targeting Bruton S Tyrosine Kinase In Cancers Drug Development Advances Leukemia
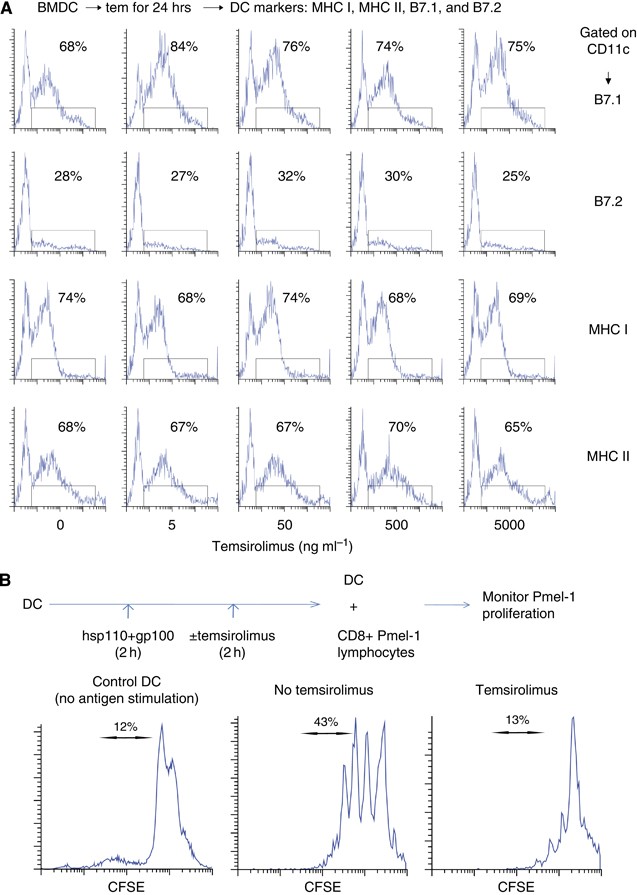
Temsirolimus An Mtor Inhibitor Enhances Anti Tumour Effects Of Heat Shock Protein Cancer Vaccines British Journal Of Cancer
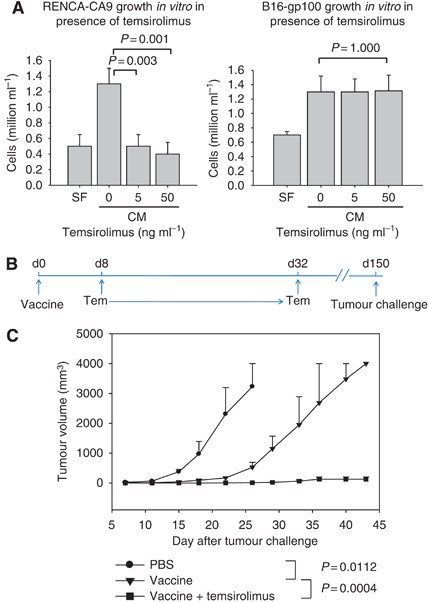
Temsirolimus An Mtor Inhibitor Enhances Anti Tumour Effects Of Heat Shock Protein Cancer Vaccines British Journal Of Cancer

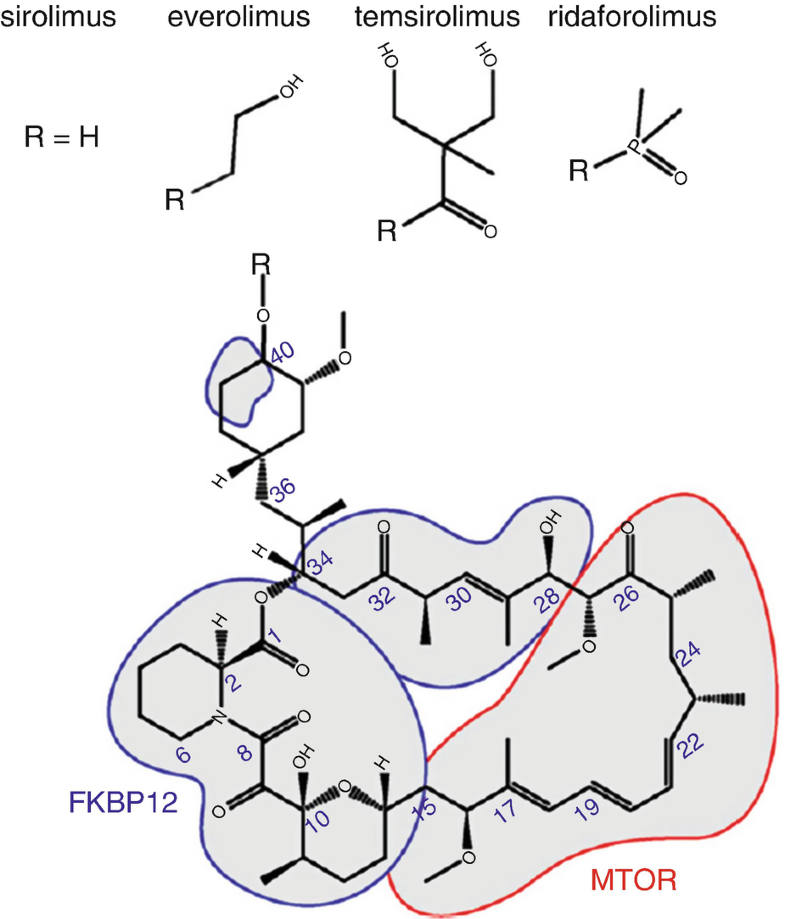
Structure Of Temsirolimus Download Scientific Diagram

Fda Approval Summary Temsirolimus As Treatment For Advanced Renal Cell Carcinoma Abstract Europe Pmc

