Temsirolimus Nebenwirkungen - Nierenzellkarzinom Beim Geriatrischen Patienten Springerlink
Tell your caregiver right away if you feel dizzy warm. The active substance in Torisel temsirolimus blocks a protein called mammalian target of rapamycin mTOR.

Temsirolimus Francais Traduction
If TORISEL is administered with drugs that.
Temsirolimus nebenwirkungen. Temsirolimus is usually given once every week. You may experience symptoms such as. Mammalian target of rapamycin mTOR inhibitors everolimus and temsirolimus are approved for the treatment of a variety of malignancies.
Temsirolimus exhibits a bi-exponential decline in whole blood concentrations and the mean half-lives of temsirolimus and sirolimus were 173 hours and 546 hours respectively. Temsirolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. We performed a meta-analysis to cal.
As a mTOR inhibitor. 2007 -----INDICATIONS AND USAGE ----- TORISEL is a kinase inhibitor indicated for the treatment of advanced renal cell carcinoma. TORISEL Kit temsirolimus injection for intravenous infusion only Initial US.
Pfizer RxPathways connects eligible patients to a range of assistance programs that offer insurance support co-pay help and medicines for free or at a savings. TORISEL when diluted contains polysorbate 80 which is known to increase the rate of DEHP extraction from PVC. 1 1 The recommended dose of Temsirolimus injection is 25 mg administered as an intravenous infusion over a 30-60 minute period once a week.
Monoclonal antibodies such as bevacizumab can block tumor growth in different ways. Temsirolimus is a substrate of the efflux transporter P-glycoprotein Pgp in vitro. To prospectively determine the efficacy of combination therapy with temsirolimus plus bevacizumab versus interferon alfa IFN plus bevacizumab in metastatic renal cell carcinoma mRCC.
It is usually given by a doctor or nurse in a doctors office or infusion center. Temsirolimus is an inhibitor of mTOR mammalian target of rapamycin. Temsirolimus exhibits a bi-exponential decline in whole blood concentrations and the mean half-lives of temsirolimus and sirolimus were 173 hours and 546 hours respectively.
Get emergency medical help if you have signs of an allergic reaction hives difficult breathing swelling in your face or throat or a severe skin reaction fever sore throat burning eyes skin pain red or purple skin rash with blistering and peeling. Temsirolimus is a substrate of the efflux transporter P-glycoprotein Pgp in vitro. Drug-Transport Systems - P-glycoprotein.
See full prescribing information for TORISEL. This complex then blocks mTOR. Temsirolimus is an inhibitor of mTOR.
MTOR is a serinethreonine kinase which plays a role in the PI3KAKT pathway that is upregulated in some tumors. Drug-Transport Systems - P-glycoprotein. Others find tumor cells.
TORISEL Quick Finder. Die Informationen dürfen auf keinen Fall als Ersatz für professionelle Beratung oder Behandlung durch ausgebildete und anerkannte Ärzte angesehen werden. CCI-779 is a potent inhibitor of mTOR with IC50 values of 06 07 07 and 50 nM for BT-474 MDA-MB-468 SKBR-3 and MCF-7 cells respectively 1CCI-779 is an ester derivative of rapamycin and has improved pharmaceutical properties.
Temsirolimus injection is a kinase inhibitor indicated for the treatment of advanced renal cell carcinoma. MTOR inhibition blocks the translation of genes that regulate cancer cell proliferation. Temsirolimus is an ester analog of rapamycinTemsirolimus binds to and inhibits the mammalian target of rapamycin mTOR resulting in decreased expression of mRNAs necessary for cell cycle progression and arresting cells in the G1 phase of the cell cycle.
In the body temsirolimus attaches to a protein inside cells to make a complex. TEMSIROLIMUS injection for intravenous use. Temsirolimus side effects.
Temsirolimus binds to an intracellular protein FKBP-12 and the protein-drug complex inhibits the activity of mTOR that controls cell division. Dieses Arzneimittel muss langsam verabreicht werden und die Infusion kann bis zu 60 Minuten dauern. TORISEL safely and effectively.
Nebenwirkungen des Wirkstoffes Temsirolimus übersichtlich dargestellt. Inhibition of mTOR activity resulted in a G1 growth arrest in treated tumor cells. Fatigue has been described with these agents as a common side effect although the overall incidence and risk remain unclear.
Some side effects may occur during the injection. Some block the ability of tumor cells to grow and spread. Since mTOR is involved in the control of cell division Torisel prevents the division of cancer cells slowing down the growth and spread of the cancer.
This should be considered during the preparation and administration of TORISEL including storage time elapsed when in direct contact with PVC following constitution. Temsirolimus comes as a solution liquid to be given by infusion slow injection into a vein over 30 to 60 minutes. If TORISEL is administered with drugs that.
In a randomized open-label multicenter phase III study patients with previously untreated predominantly clear-cell mRCC were randomly assigned stratified by prior. It also results in reduced levels of certain cell growth factors involved in the development of new blood vessels such as vascular endothelial growth factor VEGF. Temsirolimus wird in der Regel einmal wöchentlich verabreicht es sei denn Ihr Krebs macht Fortschritte oder Sie haben schwerwiegende Nebenwirkungen dieses Arzneimittels.

Effektive Immuntherapie Des Melanoms Ansatze Zur Wirkung Ohne Nebenwirkung Youtube

Universitatsklinikum Heidelberg Die Temsirolimus Studie

Temsirolimus Wirkt Auch Bei Zns Lymphomen Im Gehirn Gesundheitsstadt Berlin

Nierenzellkarzinom Beim Geriatrischen Patienten Springerlink

Tumortherapie Schadigt Noch Nach Jahrzehnten Das Herz Rosenfluh Ch

Temsirolimus Torisel 174 86 2007 Pz Pharmazeutische Zeitung
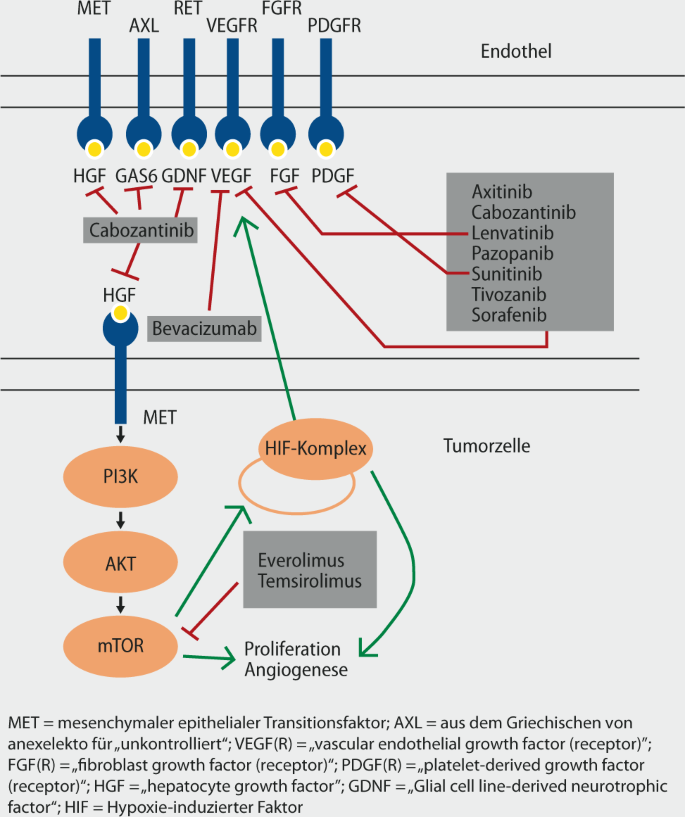
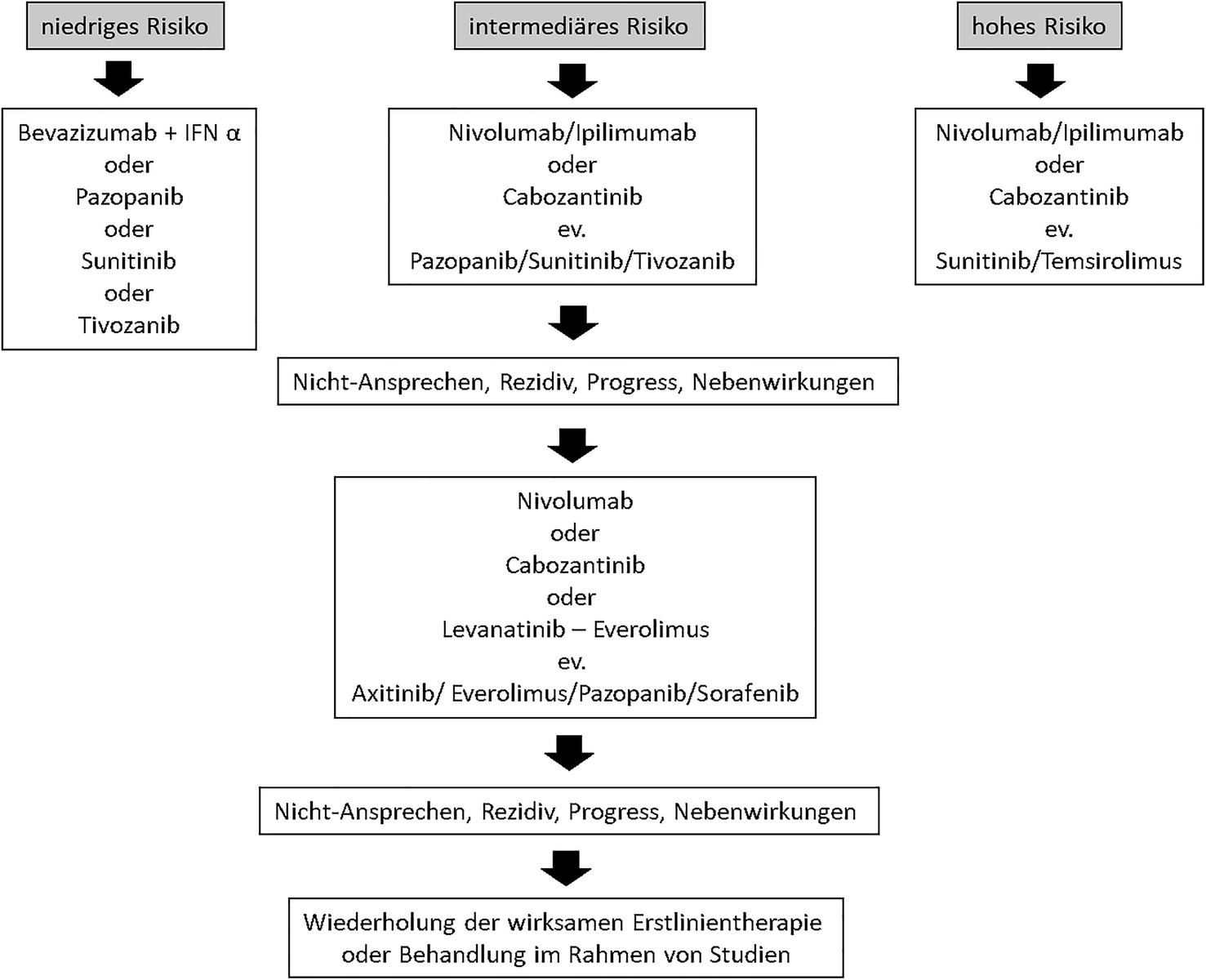
Aktuelle Zielgerichtete Therapie Des Rcc Springerlink
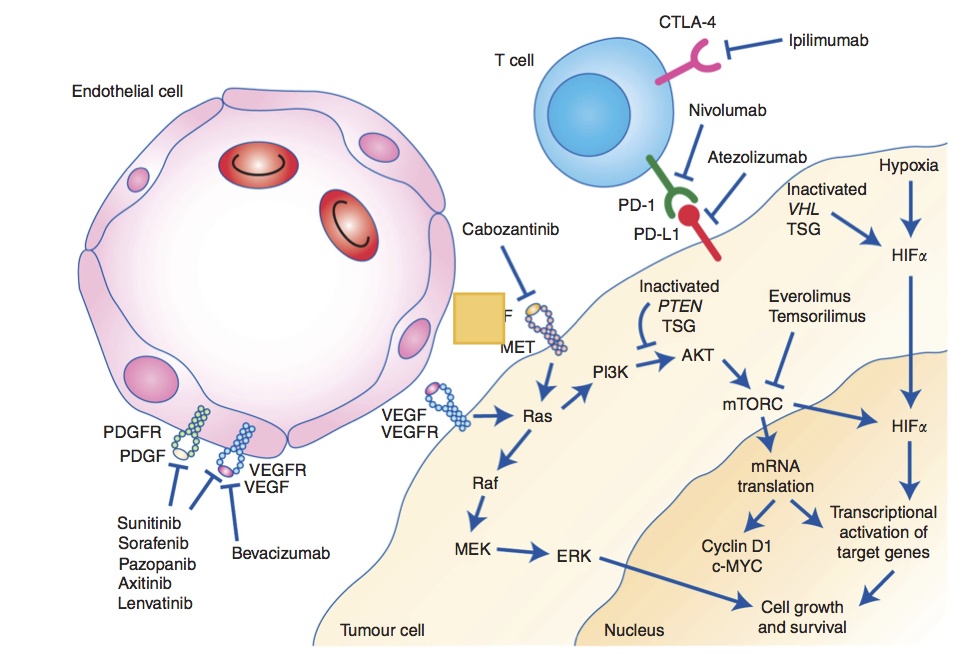
Pharmacotherapy Of Metastatic Renal Cell Carcinoma Mrcc 2017 Update Military Medicine Worldwide


